
There are 11 of them, because remember there are 20 total Amino acids. Here we'll write external Hydrocarbons because we'll go and on the next page I have here, I have all the polar R-Groups. If you think off Hydrocarbons in your R-Group basically that tells you that it will be non-polar. Then over here you have a Sulphur here in Methionine but all the outsides end up having Hydrocarbons and so that's why that's non-polar. Then Isoleucine is an Isomer of just Leucine and so also have Hydrocarbons, so that's non-polar. But the NH is embedded in a ring here, and so what happens is, this is mainly non-polar especially since the Benzene ring is super bulky. You go to Tryptophan which you find happy turkey, and basically you have pretty much all Hydrocarbons with the exception of the NH that you have here. The whole of the Hydrogens are on the outside they're all non-polar because they have the same charge. Just in case you forgot a Hydrocarbon, just made up of a Carbon and a Hyrdrogens.
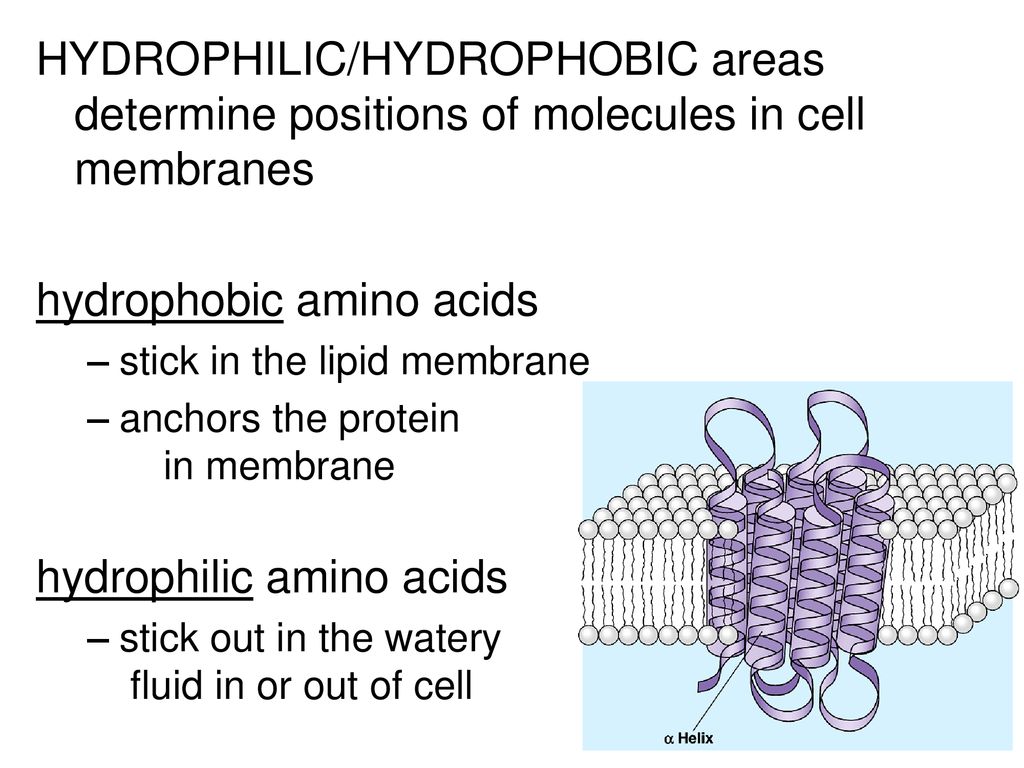
#Where are hydrophobic amino acids found plus
You go to Phenylalanine you have a Hydrocarbon plus you have the Benzene ring which is also a type of Hydrocarbon. Go to Valine, here's your R-Group, also Hydrocarbons. So Hydrocarbons would tell you that it's also non-polar. And you have basically a Hydrocarbon chain again this time it's a ring. Then now we take a look here and in Proline, you have basically these right here.
Since they all have the Hydrogens there, they all have the same charge, so they're non-polar. They all have basically similar charges they have there and what other bond is the Carbon. And we take a look here we have CH3 which is some where to Methane and if you notice Carbon only bond with the Hydrogens around it that the Hydrogens are in the back. We go to the right to the next one, Alanine. Remember non-polar means a Hydrophobic which means they don't attract to water. That's one way to obviously to figure out that that's non-polar. In the first one that we have here we have Glycine and the R-Group is just a Hydrogen. What I'll do is I'll box the R-Groups for you, and then I'll show you how it's non polar. In our first one,if you count them up we have, 9 total Amino acids that have non-polar R-Groups. As you know from way back when you studied about polar and non-polar, non-polar means that they have all the same charge or basically they don't have different charges on different ends. This first set that I'm going to show you, are all the non-polar R-Groups. So here are some tips for figuring out if an R-Group in an Amino acid is polar or non-polar. Identifying Amino acids can be difficult.


 0 kommentar(er)
0 kommentar(er)
